State of the art research
What is CRISPR genome editing and why is it so revolutionary?
The CRISPR technology allows the one-step generation of an artificial endonuclease (CRISPR/Cas9) that is able to cut the genomic DNA at a single site defined by the user, creating a double-stranded DNA break (DSB). This DSB will then be repaired by an
error-prone DNA repair mechanism called non-homologous end-joining (NHEJ, Fig. 1), creating different types of mutations around the cut site.
This method is ideal for the inactivation of genes and is already widely used for gene inactivation in cell lines and animals. It is more complicated to introduce a defined genomic change by CRISPR technology. In this case the genomic DNA has to be cut as close as possible to the site to be mutated and a DNA repair template has to be provided, where the DNA to be introduced is flanked by DNA identical to the target site. Then another DNA repair mechanism, called homology directed repair (HDR), will replace the endogenous wild type DNA sequence by the foreign DNA sequence of the repair
template (Fig. 1).
This precise genome editing can be used to introduce point mutations, to repair gene defect in human patients, but also to insert larger DNA sequences as for example fluorescent reporter genes into cell lines or animals. The preparation of such CRISPR/Cas9 endonucleases is extremely easy and cheap, cutting efficiency is high and unwanted off-target effects appear to be low. Not surprisingly, CRISPR technology is considered to become the greatest technological improvement in biomedical research since
the invention of the polymerase chain reaction 25 years ago and pharmaceutical companies as well as academic research are eager to apply it.
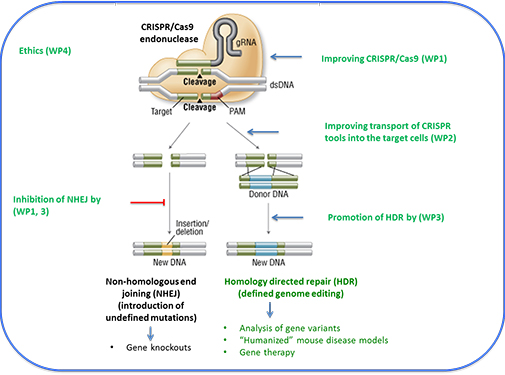
Fig. 1. Optimization of CRISPR genome editing efficiency. The CRISPR endonuclease Cas9 cuts the genomic DNA at a site defined by a short RNA sequence. The cut can be repaired by introduction of foreign DNA through homologous directed repair (HDR) or by nonhomologous end joining (NHEJ).
